In the last few years Zhiyong and I have talked a lot about “top down” petroleum systems, analysis (e.g. He and Murray, 2019), one aspect of which is “geochemical inversion”. Petroleum is a natural material containing 100’s of thousands of individual compounds, mostly hydrocarbons. Although the composition is complex it is not random: it encodes signals inherited from the original organic matter as well some related to thermal or biological processes during or after formation. Geochemists interpret this to provide information on the origin and history of a reservoir fluid.
However, I get
nervous when the results of geochemical inversion suggest complicated charge
histories which are not matched by an equally complicated geological/tectonic
history. Recently I reviewed a paper which suggested eight discrete charge
events had contributed to the fill for a cluster of fields. The corresponding
burial history looked fairly simple so it was hard to imagine how charge could
be anything but smooth and continuous in the area. I have a feeling that
interpretations like this arise from a lack of recognition of how heterogeneous
fluid compositions can be, even in well-connected reservoirs, charged slowly
and continuously by a single source rock.
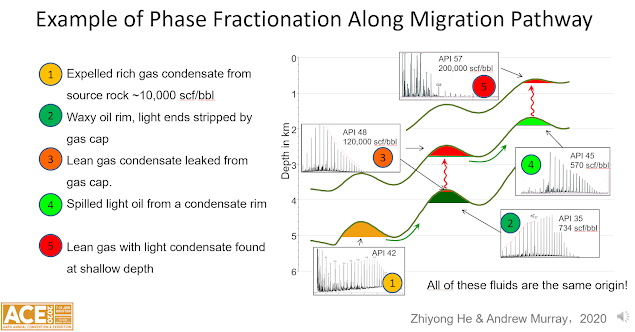
In an AAPG talk last
year (Murray and He, 2020) we noted that it is quite common for the oil
underlying a gas cap to be undersaturated with gas. This shouldn’t be
surprising, given that the rate of filling – which is limited by the rate of
kerogen maturation during burial - is of the same order of magnitude as the rate
of diffusion driven mixing. If the
kerogen organofacies is not uniform (normal for fluvio-deltaic and
fluvio-lacustrine source rocks in particular), and fluids are not fully mixed,
we would not expect the fluids in the reservoir to be uniform either.
Furthermore, since fluids are expelled over a source rock maturity range from ~
0.7 to 1.3% Ro (vitrinite reflectance), we would not expect to find a uniform “maturity”
signal in most oils either, whether it is based on methylphenanthrene isomer
ratios or gasoline range ratios or whatever.
My experience of
reservoir geochemistry studies, where samples from multiple depths, units and
wells within a single field are examined, mostly confirms these expectations: A
lot of fields I have looked at do not contain well-mixed fluids, independently
of any physical compartmentalisation that may exist. This is hardly a new observation:
England (1990) commented on it in relation to the Forties field for example.
Indeed, it is more surprising when reservoir fluids are found to be well
mixed. I have seen examples of this too
though and it seems to be when (a) geometric factors in migration homogenise
fluids before or during their arrival at the trap or (b) thermal disequilibrium
accelerates density overturn via convection and therefore mixing. My colleagues
and I described the latter process in respect of the remarkably well mixed
fluids in the Sunrise gas-condensate field (James et al., 2010). Well-mixed
fluids are also quite common in fractured carbonate reservoirs where mixing
pathways are short due to polygonal fracturing.
My point in mentioning the unmixed fluids is that geochemical inversion
studies frequently base their conclusions only one sample from each particular
field or reservoir, without taking this into account.
A specific example of geochemical inversion is the interpretation of patterns of biodegradation in terms of reservoir temperature vs. charge history. Biodegradation, which occurs at temperatures lower than about 80 °C, has easily recognisable effects on oil. The most characteristic feature is the complete or partial loss of the n-alkanes (also called n-paraffins). These straight-chain compounds are easily assimilated by bacteria and gas-chromatograms of biodegraded oils show their depletion relative to the “unresolved complex mixture (UCM)” hump. Note that no new material is formed here – bacteria do not convert straight chain hydrocarbons into the branched and cyclic hydrocarbons comprising the UCM – the latter are just more resistant to attack. A chromatogram of a crude oil with complete loss of n-alkanes is shown in figure 1.
 |
| Fig. 1 Gas chromatogram of a severely biodegraded oil from the Vincent Field, Australia (Murray et al., 2013) |
A so-called “polyphase”
or “hybrid” oil is one in which it is suggested that more than one discrete
charge/biodegradation event occurred. This is usually based on the simultaneous
presence of very easily degraded and very resistant compound. An example is the
co-occurrence in an oil of n-alkanes and the 25-norhopanes, a group of
pentacyclic terpane biomarkers associated with a severe level of biodegradation
(Peters et al. 2005 and references cited therein). The n-alkanes are attributed
to a component of the charge arriving after the reservoir temperature exceeded
80 °C when biodegradation stopped. A similar conclusion is sometimes drawn when
the gas chromatogram shows prominent n-alkanes on top of a large UCM, as shown
here in figure 2.
 |
| Fig. 2 Gas chromatogram of a “polyphase” biodegraded oil from the Lady Nora Field, Australia. MCH is methyl cyclohexane, a cyclic alkane which is relatively resistant to degradation |
Back in 2005 I worked
on a heavily biodegraded oil field in the Middle East. Being onshore and shallow it had been pattern
drilled and there were a lot of samples to play with. Gas chromatograms showed
the usual UCM with n-alkanes and resolved peaks from other simple compounds
present to variable degree. There was a good correlation between API gravity
and the area of GC-resolved peaks relative to the UCM, as shown in figure 3.
 |
| Fig. 3 Correlation between the total area of resolved peaks (relative to the UCM) and API gravity of oils from a large oil field in the Middle East region |
This correlation was
useful in estimating the API and the viscosity (by another correlation) of
fluids for which there was insufficient sample for direct measurements. However, in order to predict bulk properties
away from well control, we needed to understand the factors controlling the extent
of degradation. Because there were
spatially coherent differences in the degree to which light vs. heavy “fresh”
charge overprinted the UCM, I concluded, at the time, that there were multiple
stages of charge and degradation. The problem was that the burial history was
simple and charge should have concluded more than 100 Ma before present. At the time, I thought there must have been
things in the charge history – perhaps to do with “motelling” or some other
migration-related process - that were
not captured in the charge model. However, I revisited the report recently and realised
there is another possibility. It goes like this…
Several studies have shown that heating of the asphaltene fraction of a heavily biodegraded oil can release fresh oil, complete with the original complement of n-alkanes (Snowdon et al. 2016 and references therein). Asphaltenes are macromolecules with a composition and molecular structure similar to that of the kerogen from which they were derived (Snowdon et al. , 2016). Laboratory pyrolysis of asphaltenes is thus akin to the artificial maturation of kerogens. Figure 4 shows gas chromatograms (and density, viscosity) of a heavily biodegraded oil from a field in the Middle East region, before and after heating at 300 °C for 12 days and at 350 °C for 10 days. The thermal stress from these two heating regimes is equivalent to a vitrinite reflectance of 0.8 and 1.3% respectively.
 |
| Fig. 4 Gas chromatograms for the original oil from a large oil field in the Middle East region and after heating as shown. I.S. is the “internal standard” added to assist quantitative analysis |
If we can do this in
the laboratory, why would it not also happen in nature as a reservoir
containing biodegraded oils is buried deeper?
Let’s consider such a reservoir which is continuously buried so that the
temperature increases from 80 °C to ~ 120 °C over a period of
about 20 Ma. Using the kinetics of asphaltene conversion from laboratory
studies, we can estimate that about half of the mass of asphaltenes would be
converted to “fresh” oil. The
chromatogram, perhaps like that in Fig. 4B, would show a “polyphase” character,
without the requirement of any new charge arriving from the source rock after
biodegradation ceased.
What if the reservoir
is not heated as high as 120 °C? Could we still get an apparently polyphase oil?
I believe so: Some studies (see Snowdon et al., 2016 and references cited
therein) have shown that the source of fresh oil in asphaltene heating studies
is not only pyrolysis (i.e. the breaking of high-energy covalent bonds).
Rather, the cage-like molecular structure of asphaltenes appears capable of
encapsulating some of the original oil and preventing it from being biodegraded
in the first place. This oil can be released by thermal disruption of the
asphaltene clusters at temperatures lower than those required for pyrolysis.
Figure 5 shows before and after heating chromatograms for a crude oil which had
been severely biodegraded at the surface (following an oil spill). The
conditions used, 320 °C for 2 days, create a level of thermal stress similar
to that applied to the oil in figure 4B. However, in this case the post-heating
oil has lots of n-alkanes and only a very small UCM. I wonder how much of the
fresh oil here has been released prior to pyrolysis temperatures being reached.
 | |
| Fig. 5 Gas chromatograms for the original, biodegraded oil collected after a spill at sea and after heating at 320 °C for two days (from Oudot and Chaillan, 2009) |
In almost all cases where complex charge histories are invoked to explain geochemical anomalies, I can (at least in principle) explain them by things that happen during, normal continuous burial and supply of hydrocarbons. This doesn’t mean that the simple explanation is necessarily true - just that, in the absence of evidence for a complex burial/thermal history, we need not be as puzzled as I was back in 2005.
As with all these blog posts, I invite and indeed welcome push
back/comments/clarification. They are not peer-reviewed papers, just some observations
and thoughts from one individual.
Cheers,
Andrew Murray,
References:
England W. (1990) The organic geochemistry of petroleum reservoirs. Org. Geochem., 16, 415-425
He Z. and Murray A. (2019) Top Down Petroleum System Analysis: Exploiting Geospatial Patterns of Petroleum Phase and Properties. AAPG Search and Discovery, #42421
James B., Bailey W, Murray A., Pelechaty S., Kaiko A. and J. Li (2010) Unusual reservoir connectivity revealed by data integration at the Sunrise Field. APPEA J. 50th Anniversary issue, 349-370, Australian petroleum production and exploration association (A PDF is available from the author on request)
Murray A. and He. Z. (2020) Oil vs. Gas: What are the Limits to Prospect-Level Hydrocarbon Phase Prediction? AAPG Search and Discovery, #42513
Murray A., Dawson D.A., Carruthers D. and Larter S. (2013) Reservoir Fluid Property Variation at the Metre-scale: Origin, Impact and Mapping in the Vincent Oil Field, Exmouth Sub-basin. Proceedings of the Western Australian Basins Symposium, Petroleum Exploration Society of Australia, Perth, August 2013 (A PDF is available from the author on request).
Oudot J. and Chaillan F. (2009) Pyrolysis of asphaltenes and biomarkers for the fingerprinting of the Amoco Cadiz oil spill after 23 years. Nature Precedings. 4. 10.1038/npre.2009.2975.1
Peters K. E., C. C. Walters and J. M. Moldowan, 2005, The Biomarker Guide: Cambridge University 479 Press, Cambridge, U.K., 1155 p.
Snowdon L., Volkman J.K., Zhang Z., Tao, G. and Liu, P. (2016). The organic geochemistry of asphaltenes and occluded biomarkers. Org Geochem., 91, 3-15.