By Andrew Murray
"Top Down" petroleum systems analysis (TDPSA) includes petroleum geochemistry and, specifically, the process known as “geochemical inversion”. Given even a small fluid sample, we can extract information on:
- The organic matter type and depositional environment of the source rock
- In some cases (and only in broad terms) the age of the source rock
- Whether more than one source rock and/or kitchen area has contributed
- What alteration processes have affected the fluid in or on the way to the trap
Other applications might include:
- Estimating the average or integrated thermal “maturity" of the source rock at the time of expulsion
- Identifying separate (distinct in time) fill events
There are problems, however, both conceptually and in practice, with the last two: See here:
http://petroleumsystem.blogspot.com/2014/10/measuring-maturity-of-hydrocarbon-fluid.html
http://petroleumsystem.blogspot.com/2020/12/does-complex-geochemistry-of-oil-mean.html
The most valuable information obtained from fluid geochemistry is the organic matter type/depositional environment of the source rock. This is the strongest determinant of yield and the gas to liquids ratio (GLR). We can measure the GLR of a fluid sample (if we have enough of it) but that doesn’t necessarily tell us the “system” GLR, i.e. what came out of the source rocks in the kitchen. We need that information to assess the chance of finding oil, gas and also as an input to the probabilistic prediction of migration. Does one need to be an expert geochemist to extract this information? Well, it helps – but there is a lot a non-expert can do, as we shall see. Firstly, though, it is necessary to recognise that a few things have hampered the integration of petroleum geochemistry into TDPSA:
- A disconnect that commonly happens somewhere between the PVT lab and the geochemistry lab, leading to loss of spatial context and relation to fluid bulk properties such as GOR, API, saturation pressure etc: Most geochemical databases and many reports do not align PVT and geochemistry samples
- Traditional analytical methods focusing on the trace, higher molecular weight biomarkers in the C12+ fraction, leading to an intrinsic bias against the origin of the gas fraction when more than one source rock contributes (Murray and Peters, 2021). While analyses of the gases (C1- C5) and gasoline range (C6 – C9) hydrocarbons are often included, there is a fundamental trade-off: low molecular weight hydrocarbons are present in high concentration and thus likely representative of the bulk fluid, but offer low source discrimination. High molecular weight biomarkers are present in low (and variable) concentration but they provide more discrimination. This trade-off is summarised in the figure below.
- Lack of quantitative data and use of compound ratios rather than absolute amounts in interpretation. This causes problems when more than one source rock/kitchen is contributing and/or when biodegradation, phase separation or migration contamination occur. All of these things can hide or distort the contribution of one source over another.
- Interpretations placing too much (or too little) emphasis on individual geochemical features, without regard to their relative discriminating power.
In recent years, analytical methodology has improved to the point where it is no longer necessary to separate a crude oil or condensate into chemical classes (Saturates, Aromatics, Resins, Asphaltenes) before analysis by gas chromatography (GC) or gas-chromatography-mass-spectrometry (GCMS). This means the whole oil (C6+) can be analysed in a single run. It also facilitates quantitative analysis which, when combined with the GOR, allows true whole reservoir-fluid concentrations to be calculated. GeoMark Research have always included concentrations in the C12+ liquids in their standard protocols and they kindly allowed Ken Peters and I to use the data in their global database (https://www.geomarkresearch.com/rfdbase) to assess the impact of fluid mixing on geochemical interpretations. More recently, they have instituted a combined high-resolution GC (HRGC) and triple quadrupole GCMS (QQQ) protocol to provide quantitative data on whole oil samples for a very wide range of target compounds. Even more recently they have improved the GC part of this analysis, making it "Ultra High Resolution GC" (UHRGC) with up to 190 individual compounds identified:
The GeoMark database (also helps us to assess the relative discriminating power of different geochemical parameters, as regards source rock type. (Some years ago, from 10,000+ oils in the library, I made a subset of 2843 oils/condensates which met the following criteria (a) not biodegraded (b) not very high or low maturity (c) not contaminated (d) have a complete data set available (e) are from a well-understood petroleum systems with high confidence about the identity of the source rock. GeoMark use source rock type descriptors such as “Marine Carbonate”, “Distal Marine Shale”, Lacustrine Freshwater for algal OM type sources and “Paralic”, Coal-resinitic”, Paralic Deltaic” for land plant type. These correspond to the Pepper and Corvi (1995) organofacies, A, B, C and D/E respectively (there are no “F” oils because, in the standard formulation, an F type source rock expels no oil). The distribution for each source type is shown below.
The figures below show statistics (mean, +/- 1 SD and range) for five common geochemical source parameters; pristane/phytane ratio, % sulphur, the C29 to C30 regular hopane ratio, the % C27 steranes among the C27-C28-C29 total and the ratio of C26 to C25 tricyclic terpanes. It is apparent that some of these parameters, if used in isolation, offer only weak discrimination. This is why all good interpretations use multiple parameters visualised with cross-plots or via multi-parametric statistics.
These are only five of the numerous geochemical source parameters which geochemists can use and which are described in the Peters, Moldowan and Clifford text book “The Biomarker Guide” (https://www.amazon.com.au/Biomarker-Guide-K-Peters/dp/0521039983)
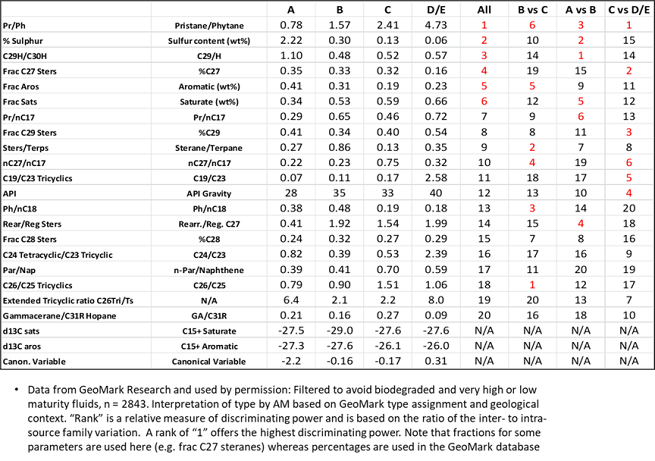
How can a non-expert know which
parameter(s) to use? The table below gives the median values for each source
rock type and ranks the parameters from most (1) to least (20) discriminating
power:
We start with the “All” column and the first parameter (Pr/Ph) and find the source type with the median value closest to that of the sample in question. Then we do the same for the next parameter down the list (% sulphur) and so on until parameter six. By then, the source type should be clear but, if it’s not, we can continue down or look at the “B vs. C” column and note that the strongest parameter for distinguishing between a “B” and “C” type source rock is the C26/C25 tricyclic terpane ratio. The second strongest discriminator in that column is the ratio of total steranes/terpanes (some reports use steranes/hopanes). The same process applies if we are trying to choose between “A” and “B” or between “C” and “D/E” types. Note that values are given in the table for median carbon isotope ratios but these are not ranked for discriminating power. The parameter notation is based on the standard GeoMark summary sheets. If data from other laboratories are used, they will generally correspond and in most cases differences in analysis protocols etc will not make much difference to the process. Nevertheless, it is obviously better, if possible, to have your sample analysed by GeoMark. For those who subscribe to the OILS library, that will also allow a search for geochemical analogs, which can be illuminating. This is one of the benefits of subscribing to the GeoMark OILS library: it provides the perspective that is often absent from narrower regional studies but is essential for solid interpretations.
Of course, nature is a continuum and source rocks from transitional environments, as well as oils which are mixtures, may emerge from this process as “B/D/E” or “C/D/E” etc. The latter is common for deltaic petroleum systems where lacustrine and fluvio-deltaic source rocks occur in association, the Pearl River Mouth Basin in China being a good example.
The focus here has been on the typical parameters used by petroleum geochemists. However, the process should not be separated from geological and engineering data: If an oil types geochemically to a “C” type source rock but is a single-phase fluid with a GLR above 2000 scf/bbl it’s likely there is a separate source of gas. That might be a D/E (or F) source rock, biogenic gas, residual liquids cracking in the source rock or in-reservoir oil cracking. Gas geochemistry then comes into play. We find that the number and type of distinct source families can often be determined using just four parameters from the PVT report: GLR, API of the liquids, saturation pressure, whole reservoir fluid gas wetness - and one geochemical parameter: good old pristane/phytane. .
References cited:
Murray A.P and Peters K.E. (2021) Quantifying multiple source rock contributions to petroleum fluids: Bias in using compound ratios and neglecting the gas fraction: AAPG Bulletin, v. 105, no. 8 (August 2021), pp. 1661–1678
Pepper A.S. and Corvi P.J. (1995) Simple kinetic models of petroleum formation. Part I: Oil and gas generation from kerogen: Marine and Petroleum Geology, v. 12, no. 3, p. 291–319